Data & Analytics Solutions
Actionable Insights to Accelerate Value Delivery
Drive operational excellence and increase the speed, efficiency, and quality of your data.
Purpose-Built, Scalable Platform
Customized analysis and reporting to meet the unique needs of stakeholders across the drug development lifecycle.
The UBC Evidence Engine creates a data ecosystem integrating our real-world data platform with our visual, interactive UBC Analytics visual dashboards. All of this contained within a centralized, online platform serving as the project’s central analytics hub. Customizable and scalable to meet the needs of your program, therapeutic area, and product portfolio, the platform provides a powerful comprehensive toolset to optimize project operations and performance.

UBC Evidence Engine
UBC’s Evidence Engine source-agnostic data solution utilizes a priority processing framework to integrate and harmonize data with any data source from any partner on any schedule. Serving as the foundational component for our data-driven technology, this platform includes our analytics capabilities and other evidence generation technologies, with the patient privacy and storage capabilities necessary to meet applicable regional requirements.
- The ability to process and store any data from any format via any data delivery channel allows maximum data integration flexibility.
- Reduces the burden of delivering data and speeds up the data exchange process.
- Evidence Engine does not require a standard data specification for data exchanges.
- The patient identity management module utilizes a vendor-agnostic process to assign a unique universal token for all patients enrolled in the registry or trial.
- Standard enrollment process that captures patient PII capture process that aligns with all major security and privacy regulations.
- Tokenization provides the ability for universal patient mastery across all studies, trials, and programs (including Hub, REMS, and PV programs).
- Ability to link study data partners and real-world data providers without requiring the sharing of patient personal information between the two parties.
- The ability to perform full data verification, standardization, and edit check processing during data processing. In addition, workflow processing occurs after notification of any data processing issue.
- Import retains full traceability of data to source along with full historical retention to support longitudinal reporting and analysis.
- All data imported and stored at a transactional level in the warehouse, allowing for maximum flexibility and reusability of data in operational reporting and analytical models.
- All processing of data is fully 21 CFR Part 11 compliant.
UBC’s Data Warehousing Capabilities
UBC Analytics
Integrated data analytics platform.
UBC Analytics, a holistic visual, interactive toolset, leveraging the UBC Evidence Engine data framework, provides access to curated program data, enabling stakeholders to inform and optimize the patient journey.
- Program-focused analytics to inform operational delivery.
- Fit-for-purpose, incorporating unique workflows based on program requirements.
- Data source-agnostic, integrating all program data in a centralized framework via UBC Evidence Engine.
- Automated, customizable data alerts, sent via email, including visual, exploratory dashboards and detailed listings of the full suite of available data tools.
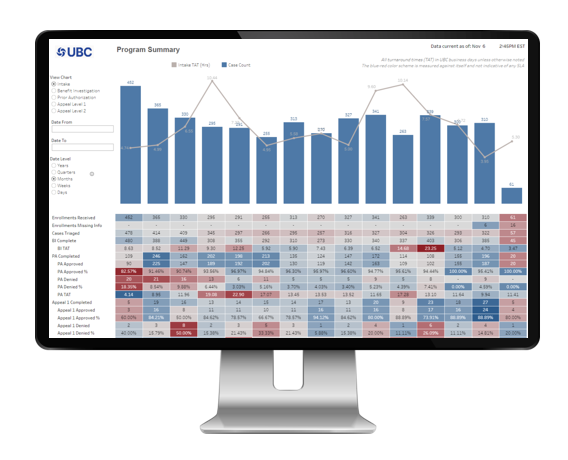

Integrated Data Analytics Platform
Learn More About UBC's Data & Analytics Platform
Interested in learning more about how UBC’s data and analytics platform can help you make smarter, evidence-based decisions?





