Advanced Software Solutions to Ensure Patient Safety
UBC provides world-class validated software solutions to help sponsors mitigate risk and demonstrate product safety.
Unlike many off-the-shelf software solutions, UBC’s solutions are purpose-built, focusing on the product’s risk-benefit profile specific to Risk Evaluation and Mitigation Strategies and Pharmacovigilance programs to meet regulatory agency requirements.
UBC is able to fully customize our proprietary platforms and applications to cater to the nuances of each unique REMS and Pharmacovigilance program, all while adhering to Regulatory requirements and maintaining audit and inspection readiness.
Reduce complexities of REMS programs from inception to operations.
UBC’s industry-leading REMS technology delivers highly configurable, regulatory compliant, and expertly designed options to optimize REMS design, delivery, and ongoing patient and healthcare prescriber confidence.
Informed by unique proven experience and tenure in the REMS space, UBC has developed a fully customizable configurable, validated platform that expedites time to build, enhanced operator efficiency and quality, with multiple system attributes that help reduce resources associated with administering a REMS program. The platform offers a wide range of capabilities affording stakeholders confidence while easily navigating specific REMS components. UBC’s proprietary technology includes faster access to therapy, reduced enrollment time for patients and prescribers, and appropriate of product dispensing via specialty pharmacies, distributors, and wholesalers.

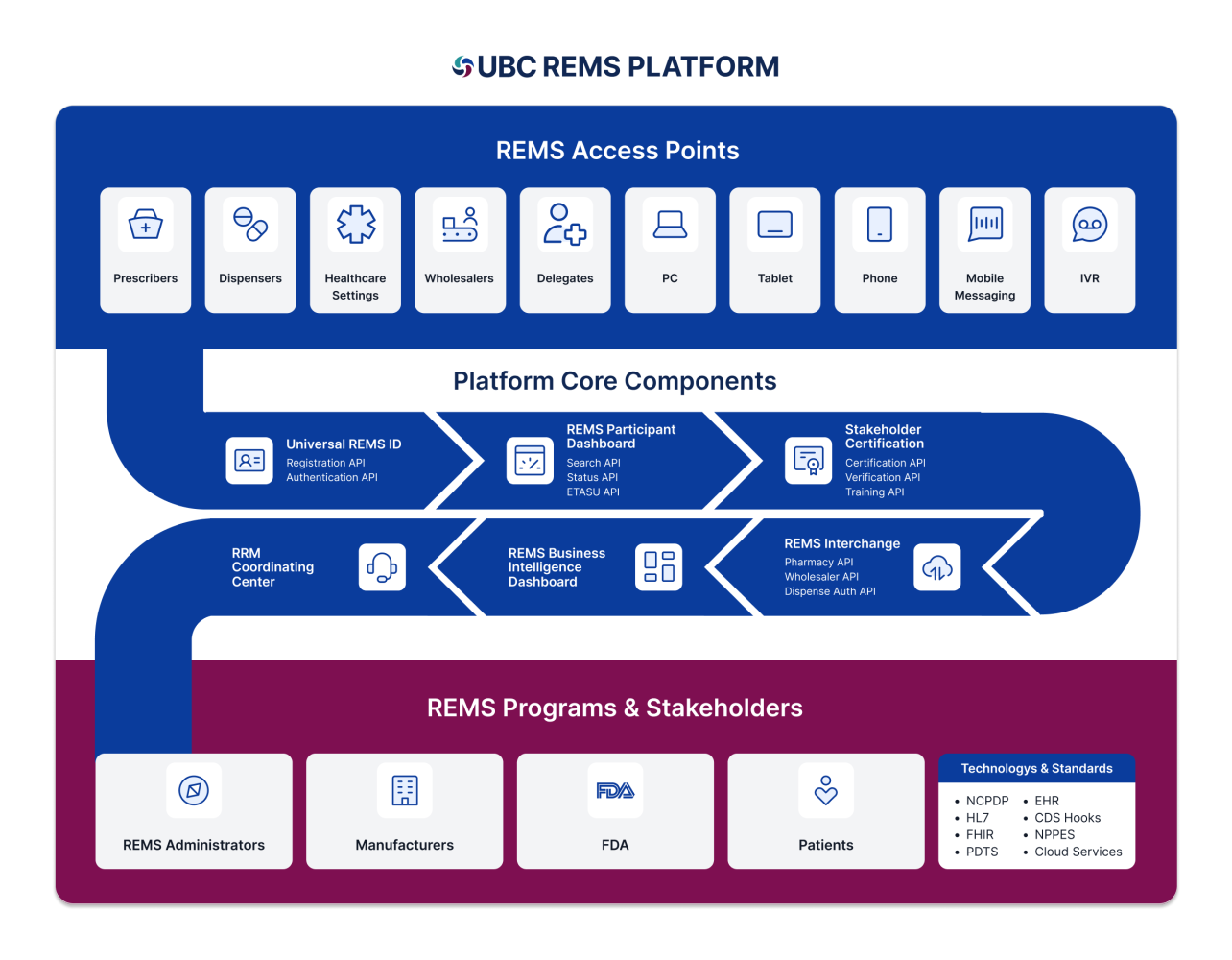
UBC REMS Platform
UBC’s unique proven tenure and expertise in the REMS space allows us to offer a myriad of data integrations with healthcare systems including electronic health record (EHR) systems, pharmacy networks lab data, hub/patient services, safety databases product sample integration, interactive voice response, and data exchange with a wide range of aggregators. The platform can federate with external parties, simplifying the authentication process for REMS participants. Our comprehensive approach guarantees that our REMS websites adhere to the highest standards of quality and regulatory requirements while performing exceptionally in the face of audits and inspections.
Core capabilities include:
- Innovative, secure, and responsive public-facing REMS websites and coordinating center portals encompass all the essential Elements to Assure Safe Use (ETASU)
- Automated system for inbound and outbound data exchange to reduce stakeholder burden and ensure secure, timely, and safe information sharing
- Secure API options allow seamless integration with the REMS system for generating a REMS dispense authorization and other necessary data exchange
- Optimized and streamlined onboarding process for specialty pharmacies and distributors
- Validated coordinating center application assisting healthcare providers in their tasks, supported by email fax, or phone communication channels and data for FDA assessments.
- Single Sign-On (SSO) interface, enhancing
ease of access and facilitating participation by healthcare providers - Efficient data migration and sanitization for legacy REMS programs migrating to UBC’s system along with seamless user account creation to support legacy administrators
- 21 CFR Part 11 compliant and validated and HIPPA compliant.
REMS Platform Expertise
* From 2018 to Q2 2023
UBC Epidemiology & Safety Software
Investigate, detect, and evaluate safety signals efficiently with any observational research database.
UBC’s epidemiology and safety software is a proven analytical platform that performs rapid descriptive analyses on administrative claims and electronic medical record (EMR) data.
The platform enables stakeholders to rapidly answer scientific questions related to epidemiology, drug safety, pharmacovigilance, and risk management without the need for customized programming:
- Comorbidity and concomitant medication profiles
- Disease incidence and prevalence
- Active drug safety surveillance and detection
- Risk assessment


UBC Medication Cost & Utilization Software
Real-time comparative effectiveness analyses and insights.
UBC’s medication cost and utilization software allows users to explore patterns of healthcare use and the associated costs in a user-defined cohort. Results provide information on the healthcare experience for a study population in defined timeframes.
Healthcare researchers can rapidly query large, complex healthcare databases, providing the ability to answer key cost-effectiveness questions such as the impact of a new treatment, burden of illness, utilization by payer plan, trends in healthcare over time, and physician specialty.
UBC Knowledge Survey System
UBC’s Knowledge Survey System supports the management and analysis of patients’ and prescribers’ self-reported knowledge, attitudes, and behaviors associated with serious risk and safe use conditions of a product.

UBC Pharmacovigilance Platform
Purpose-built software solutions to drive efficiency, accuracy, and quality of a pharmacovigilance program through the automation of critical pharmacovigilance business processes including real-time signal detection and management, literature review, safety case processing and reporting, as well as real-time sponsor-facing dashboards.
Pharmacovigilance Automation
UBC’s custom-developed technology is purpose-built and tailored to drive efficiency, improve speed of processing, and increase quality.
- Drive efficiency and automation through practical real-world solutions that reduce labor associated with case intake
- Improve speed of case processing through automation of repetitive tasks
- Increase quality by allowing our clinical staff to focus on higher-end activities and reducing the incidence of human error through robustly tested technology algorithms
Automated Mailbox Management
Monitors PV mailbox for inbound safety report forms. Automates the workflow with unique identification, data extraction, & case tracking. Automates email acknowledgement & reconciliation.
Book-In Data Entry
Automation algorithms maps safety report form into the standard E2B format and then into Argus for automated case creation. Customized for client-specific documents and integrated with UBC’s certified translation vendor.
Automated real-time duplicate checks.
Work Assignment
Algorithm assigns cases to CP team.
Considers work hours, geographic location, prioritized based on program requirements (IRD, reportable country, etc).
Easy access to artifacts: original email, source documents.
Weekly Reconciliation Report
Automation service tracks every e-mail received within the automated inbox for the purpose of providing a weekly reconciliation report.
Attachments and Filings
Automation tracks received files or book-in process related documents and automatically attaches them to the case in the Argus Safety Database.
Pharmacovigilance Automation Expertise
* From 2018 to Q2 2023
UBC Safety Database
UBC’s technology-enabled case processing services support clinical trials, post-marketing studies, and spontaneous reporting for various product types including drugs, medical devices, biologics, vaccines, combination products, and gene therapy supplements.
UBC selected Oracle Argus Cloud Service for intake and processing of adverse events to further enhance our safety database capabilities. Leveraging this industry-leading platform, we offer sponsors a comprehensive solution to support the full safety database lifecycle from initial set-up and configuration to data entry and case assessment, through medical review and case approval. Each sponsor is hosted within UBC’s safety database as a separate secure and private tenant.

UBC Signal Detection
Streamline access to detect and investigate safety signals.
Routine signal detection on multiple drugs within a portfolio, with many adverse events, can be time-consuming and resource-intensive. UBC’s signal detection software lets safety scientists quickly detect, investigate, and manage potential safety signals through an easy-to-use, web-based interface. Our intuitive software, bolstered by shared import and processing data sources, harnesses powerful algorithms to explore adverse events and answer critical safety and epidemiological questions.
- Standardized model to import and process all major regulatory data including FAERS, EudraVigilance eRMRs, Vigibase, Canadian data, and proprietary customer sources.
- Proprietary algorithm to track trends and analyze the effects of possible drug interactions and comorbidities in the reporting population, drastically reducing the number of false signals returned by standard algorithms.
- Ability to review and document signals of interest to provide audit history throughout the adjudication process.
- Audit logging of all signal detection runs and review statuses.
- Real-time results in compelling, configurable reports and visualizations, aligned to standard signal management processing and systems, for detailed exports for medical writing and regulatory filing.
- Auditability and traceability, fully validated for 21 CFR Part 11 and EU Annex 11 compliance.


UBC Signal Management
Accurate and auditable assessment of Safety Signals.
A critical component in drug safety is the management of product safety concerns to ensure timely, accurate, and auditable assessment of safety signals. UBC’s signal management software automates day-to-day tracking and overall management of safety signals, enabling detection and risk identification, while reducing paperwork and generating a comprehensive audit trail.
Supported by an easy-to-use, point-and-click interface, and designed to meet the needs of pharmacovigilance teams, our signal management software includes key features such as email alerts, performance reporting, and regulatory reporting to support the submission of emerging safety issues (ESI) to the European Medicines Agency (EMA) and periodic benefit-risk evaluations (PBRER) to the Food and Drug Administration (FDA).
Learn More About UBC's Technology Safety & Risk Management Solutions
Interested in learning more about how UBC’s technology solutions can help you make smarter, evidence-based decisions?

Thank You for Connecting with UBC
.
What You Can Expect Next
.
- Leverage automation to move fast
- Always give customers a human to chat to
- Automate customer support and close leads faster

Get Ready to Change Your Business
.
Service Request

Bekki Bracken Brown
President & Chief Executive Officer
Bekki Bracken Brown serves as the President and CEO of UBC, guiding the company’s mission and values, including the improvement of access for patients to receive better outcomes. She oversees all aspects of UBC, such as operations, business growth strategy, sales and marketing, and acquisition support.
With over 20 years of industry experience, Ms. Brown brings knowledge from a successful career in senior management from her tenure at Quintiles, INC Research, and, most recently, with Syneos Health. She’s been a member of the North Carolina BIO Board of Directors since 2019. She is also a member of the Healthcare Businesswomen’s Association — Southeast Chapter and CHIEF, an organization that supports women executive leaders. Ms. Brown earned her bachelor’s degree at Duke University.



